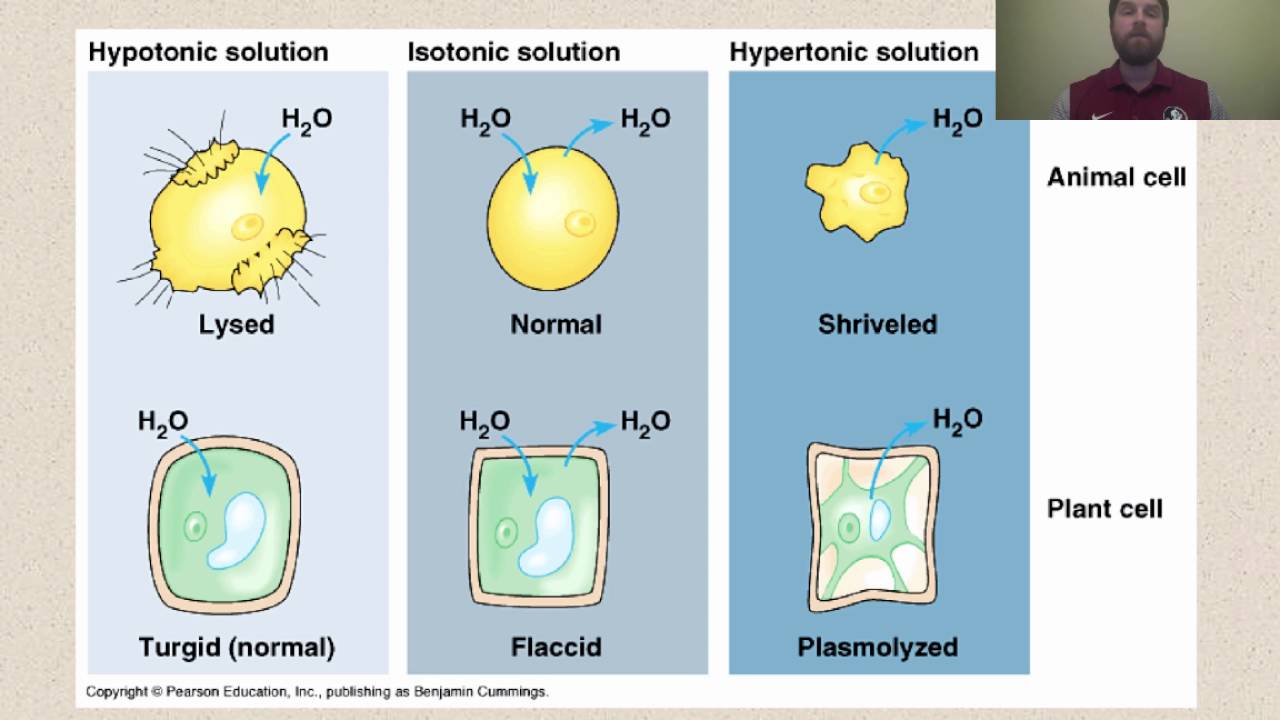
The effects of isotonic hypotonic and hypertonic extracellular environments on plant and animal cells is the same. Notice that three of the hypertonic solutions listed above contain dextrose which is a sugar.
Diabetic Emergency Coping With Type Ii Diabetes
They have the ability to dissolve in a solvent such as water h 2 o considered the universal solvent.
Types of hypertonic solutions. If a cell is placed in a hypertonic solution the cell is considered hypotonic. Watch out for pulmonary edema and fluid volume overload. Understanding the dextrose iv solutions.
There is more solute per liter in the iv solution than there is in the. Types of hypertonic solutions. Although some effects can be seen the rigid cell wall can hide the magnitude of what is going on inside.
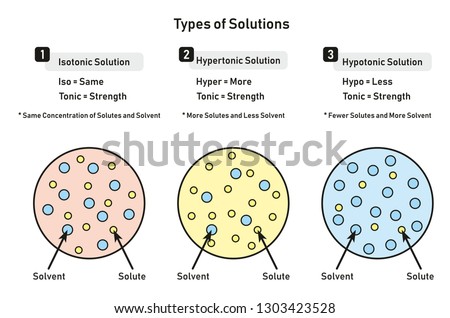
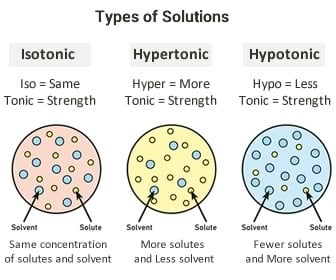
The purpose of adding sugar is to provide extra calories to the patient. It may seem like you d only find these solutions in a chemistry lab but that s not true. A hypertonic solution is a particular type of solution that has a greater concentration of solutes on the outside of a cell when compared with the inside of a cell.
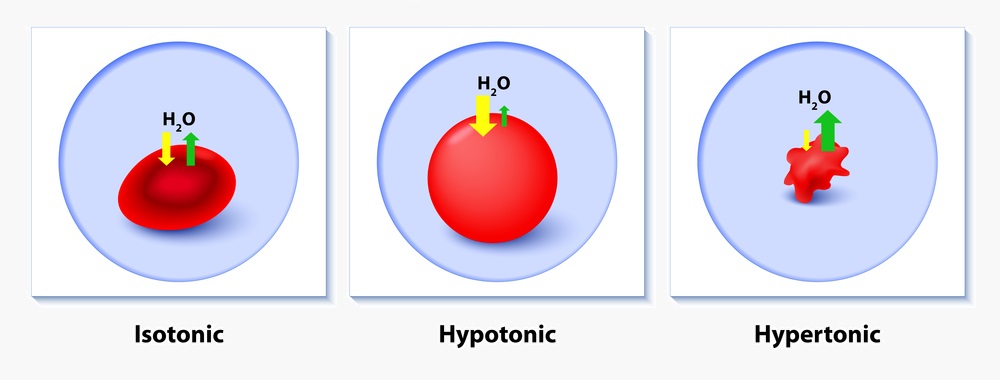
You could purchase lead types of. Hypertonic iv solutions have a greater concentration of solutes 375 meq l and greater than plasma and cause fluids to move out of the cells and into the ecf in order to normalize the concentration of particles between two compartments. This effect causes cells to shrink and may disrupt their function.
D5w 1 2 ns d5w 0 45 ns d5w 0 09 ns d5w ns 3 sodium chloride. Hypertonic refers to a solution with higher osmotic pressure than another solution. A hypertonic solution contains a higher concentration of solutes compared to another solution.
Osmosis has different meanings in biology and chemistry. The hypotonic isotonic and hypertonic solutions they are ways of naming homogeneous mixtures formed by a solute that can be classified as crystalloids and colloids thomas graham 1861. Acquire the types of hypertonic solutions partner that we come up with the money for here and check out the link.
In the group of crystalloids graham selected those that have a good ability to dissociate in water and. Giving hypertonic solutions can cause a risk for hypernatremia and volume overload. Hypertonic solutions are volume expanders.
This leads to water leaving the cell and flowing into the solution around it. The dextrose sugar is what makes these 3 solutions hypertonic. In other words a hypertonic solution is one in which there is a greater concentration or number of solute particles outside a membrane than there are inside it.
Types of hypertonic solutions recognizing the artifice ways to acquire this ebook types of hypertonic solutions is additionally useful. The opposite solution with a lower concentration is known as the hypotonic solution scientists must describe cell contents compared to the environment. Hypertonic solutions are given for hypovolemia and hyponatremia.
You have remained in right site to begin getting this info. However due to the cell walls of plants the visible effects differ.
 Hypertonic Solution Definition And Examples Biology Dictionary
Hypertonic Solution Definition And Examples Biology Dictionary
Isotonic Hypertonic And Hypotonic Solutions
 Help Hypotonic Isotonic Hypertonic Solutions Nursing Student Assistance Allnurses
Help Hypotonic Isotonic Hypertonic Solutions Nursing Student Assistance Allnurses
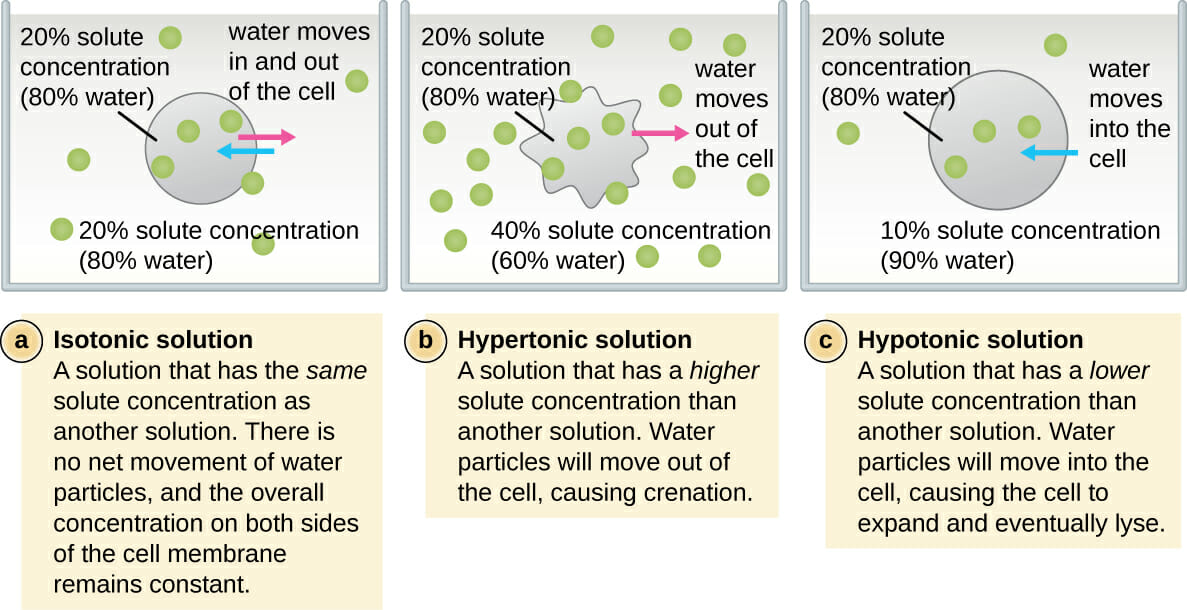 Isotonic Vs Hypotonic Vs Hypertonic Solution Biology
Isotonic Vs Hypotonic Vs Hypertonic Solution Biology
 Isotonic Hypotonic And Hypertonic Solutions
Isotonic Hypotonic And Hypertonic Solutions
Difference Between Isotonic Hypotonic And Hypertonic Definition Effect On Cells And Differences
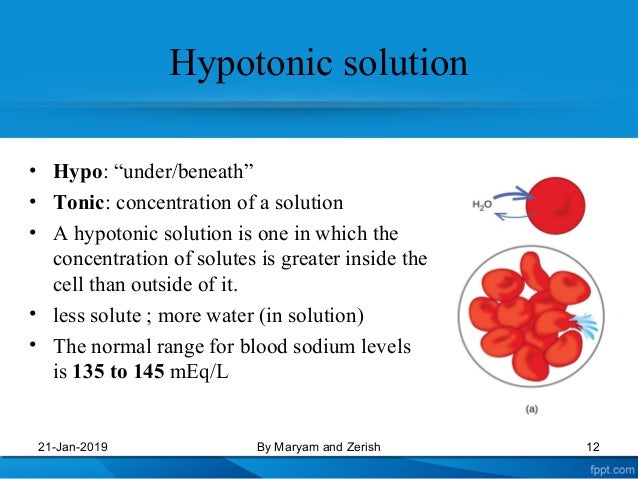 Isotonic Hypotonic And Hypertonic Solutions
Isotonic Hypotonic And Hypertonic Solutions
 Hypertonic Hypotonic And Isotonic Solutions Youtube
Hypertonic Hypotonic And Isotonic Solutions Youtube
 Iv Fluids Types Of Iv Fliuds Nursing School Survival Nursing School Tips Nursing School Studying
Iv Fluids Types Of Iv Fliuds Nursing School Survival Nursing School Tips Nursing School Studying
 Hypertonic Isotonic And Hypotonic Solution Simple Explanation Via Animation Hindi Youtube
Hypertonic Isotonic And Hypotonic Solution Simple Explanation Via Animation Hindi Youtube
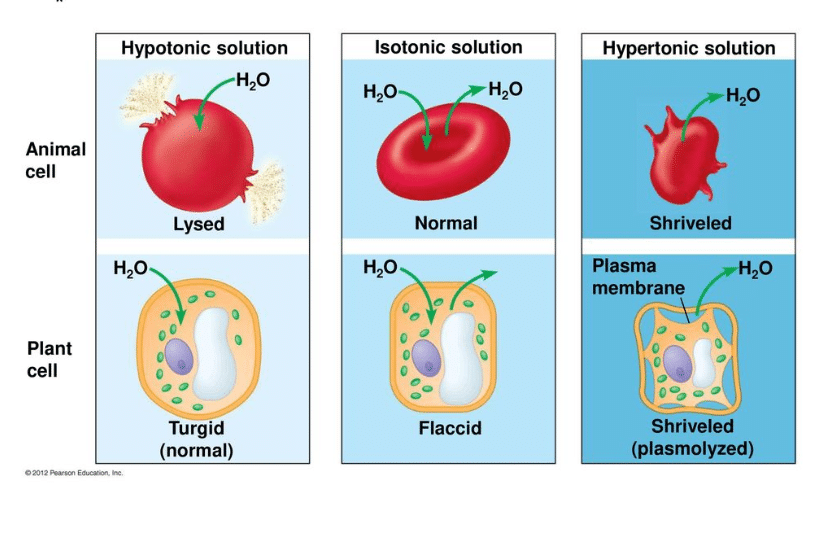 What Is A Hypotonic Solution Get Education
What Is A Hypotonic Solution Get Education