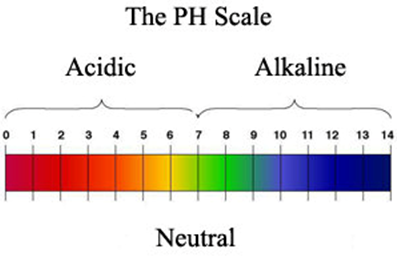
On this scale the strongest acid is 0 and the strongest alkali is 14. Ph is an abbreviation for power of hydrogen where p is short for the german word for power potenz and h is the element symbol for hydrogen.
 What Is The Ph Of Water Definition And Meaning Benefits Of Higher Ph Filkaline
What Is The Ph Of Water Definition And Meaning Benefits Of Higher Ph Filkaline
Each one unit change in the ph scale corresponds to a ten fold change in hydrogen ion concentration.
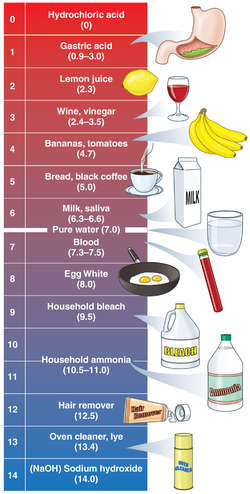
Meaning of ph level. The universal indicator turns a different colour for all the numbers on the ph scale. Updated may 07 2019 ph is a measure of hydrogen ion concentration a measure of the acidity or alkalinity of a solution. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909.
In chemistry ph piːˈeɪtʃ denoting potential of hydrogen or power of hydrogen is a scale used to specify the acidity or basicity of an aqueous solution. The ph scale is theoretically open ended but most ph values are in the range from 0 to 14. Acidic solutions solutions with higher concentrations of h ions are measured to have lower ph values than basic or alkaline solutions.
The ph scale measures how acidic or alkaline basic something is. Ph is the negative log of hydrogen ion concentration in a water based solution. A measure of the acidity or alkalinity of a solution numerically equal to 7 for neutral solutions increasing with increasing alkalinity and decreasing with increasing acidity.
The ph scale commonly in use ranges from 0 to 14. Aqueous solutions at 25 c with a ph less than 7 are acidic while those with a ph greater than 7 are basic or alkaline. Ph definition ph scale shows the range of strengths of acids and alkalis.
P otential of h ydrogen american heritage dictionary of the english language fifth edition. The term widely used in chemistry biology and agronomy translates the values of the concentration of the hydrogen ion which ordinarily ranges between about 1 and 10 14 gram equivalents per litre into numbers between 0 and 14. Ph quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
Your body works constantly to carefully control ph levels of blood and other fluids. It s a lot easier to use a logarithmic scale instead of always having to write down all those zeros. The ph scale ranges from 0 to 14.
An expression widely used in medicine of the acidity or alkalinity of a solution. Ph is the logarithm to the base 10 of the concentration of free hydrogen ions in moles per litre expressed as a positive number. The ph scale usually ranges from 0 to 14.
The body s ph balance is also called the.
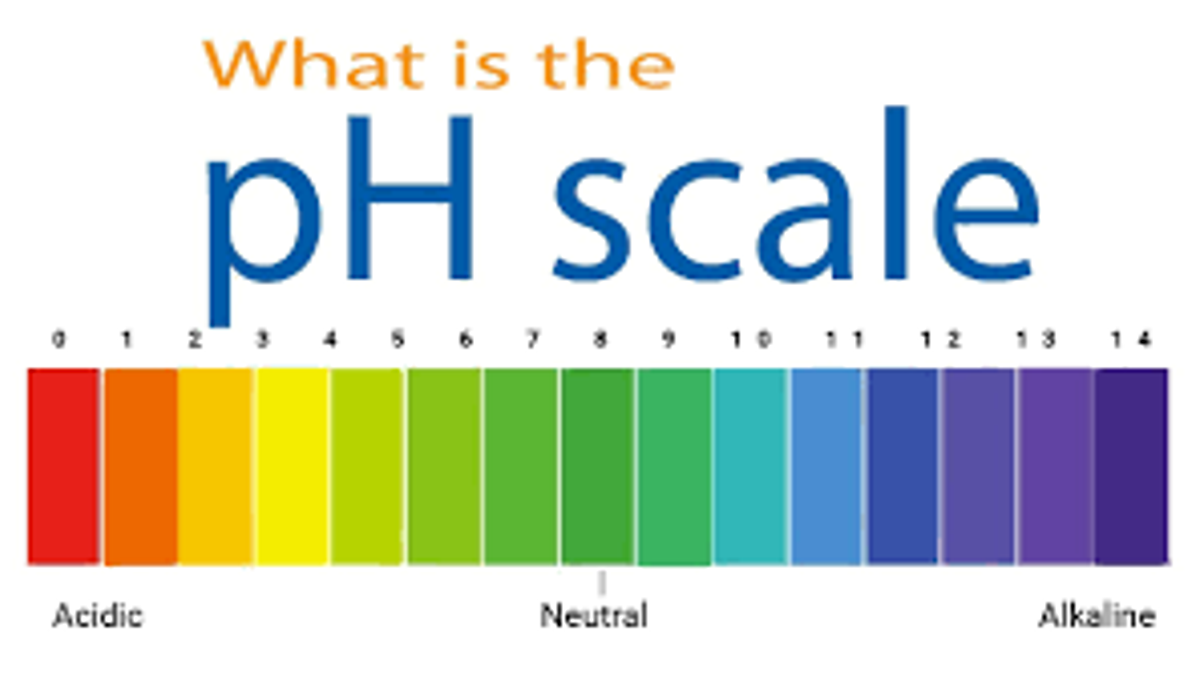 The Concept And Importance Of Ph Scale
The Concept And Importance Of Ph Scale
 Signs Causes Of Unbalanced Water Ph Levels Water Right
Signs Causes Of Unbalanced Water Ph Levels Water Right
 What Does Ph Stand For And Mean Science Trends
What Does Ph Stand For And Mean Science Trends
 Ph Scale Defined What Is Ph Jan San Consulting
Ph Scale Defined What Is Ph Jan San Consulting
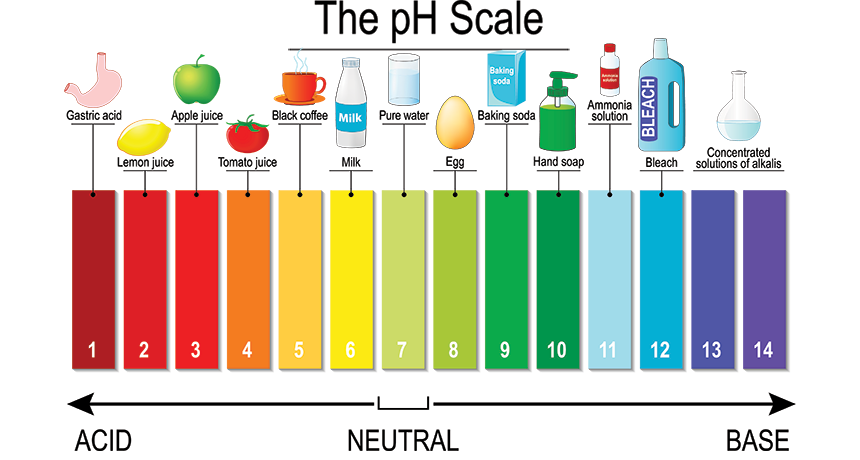 Scientists Say Ph Science News For Students
Scientists Say Ph Science News For Students
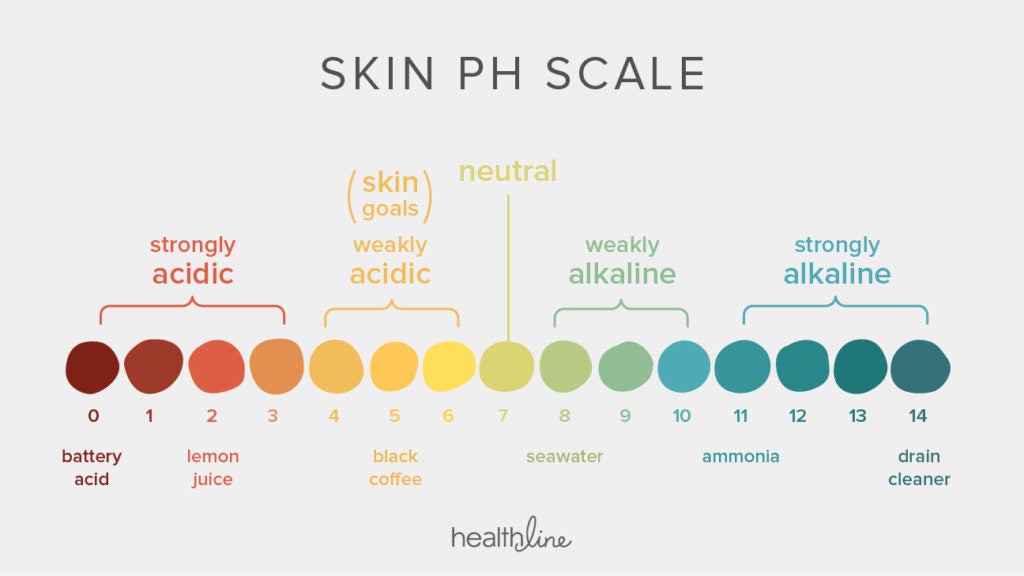 What S So Important About Skin Ph
What S So Important About Skin Ph
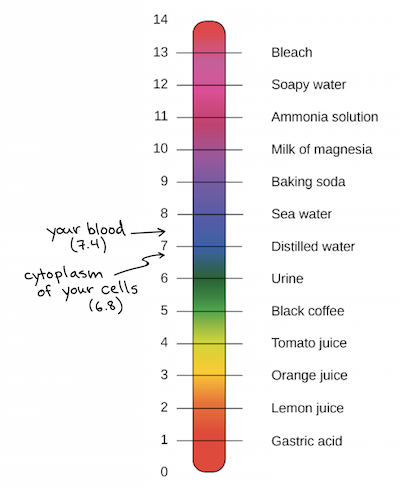 Ph Scale Acids Bases Ph And Buffers Article Khan Academy
Ph Scale Acids Bases Ph And Buffers Article Khan Academy
 Ph Level Definition Of Ph Level By Medical Dictionary
Ph Level Definition Of Ph Level By Medical Dictionary
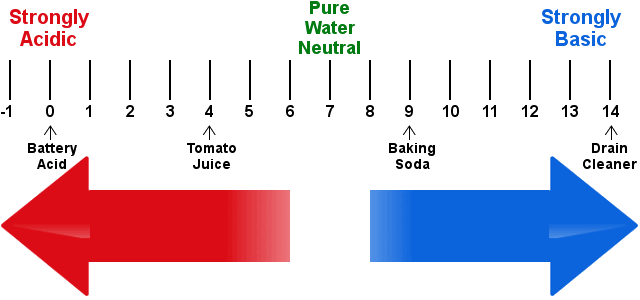 Definition Of Ph Chemistry Dictionary
Definition Of Ph Chemistry Dictionary
/definition-of-ph-in-chemistry-604605_final-5c8fac8446e0fb00017700d1.png) Ph Definition And Equation In Chemistry
Ph Definition And Equation In Chemistry
Ph Level Food Preservation Ciara